3D Printing News Unpeeled: BMF 510(K) & SprintRay Midas
Description
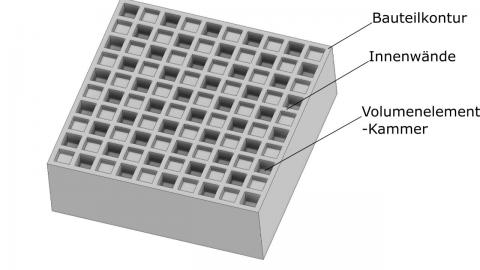
Boston Micro Fabrication (BMF) got U.S. Food and Drug Administration (FDA) 510(k) clearance for its UltraThineer micro stereolithography (PµSL) veneer solution.
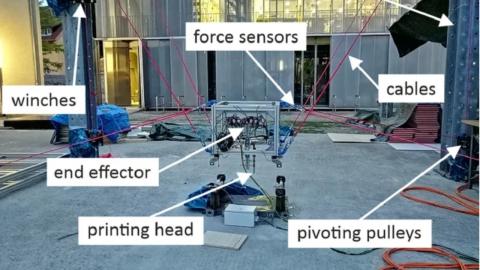
SprintRay, has released the Midas Digital Press 3D Printer. This has a vacuum-sealed Resin Capsule System, which provides easier handling for more viscous resins.
Commenting disabled.